Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Itraconazole/posaconazole analysis upgraded with voriconazole
Tri-level controls available
Custom-made internal standard
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
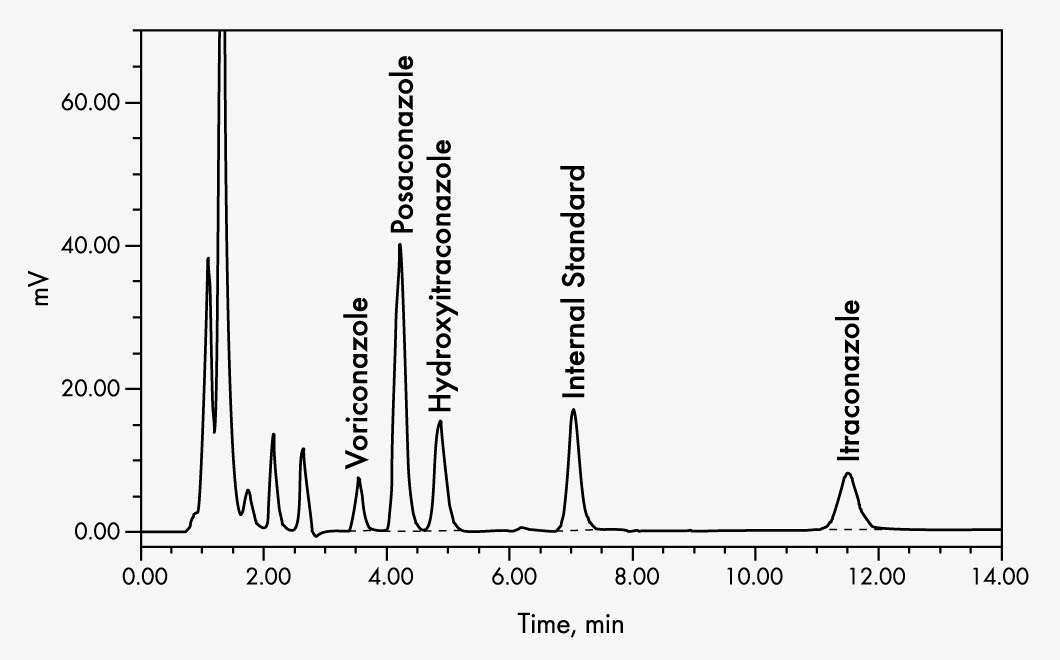

Itraconazole
Hydroxyitraconazole
Posaconazole
Voriconazole
Clinical relevance
In recent years there has been an increase in invasive fungal infections due to the growing number of people with immunosuppression. Candida and Aspergillus are the most common types of infection, but previously rare fungal infections such as fusariosis or zygomycosis are also on the rise. Options for oral antimycotic therapy have improved considerably with the advent of new drugs. These include second-generation triazoles such as posaconazole and voriconazole.
Posaconazole is used primarily in case of therapy-resistant mycoses or drug intolerances. Voriconazole is also effective against some itraconazole-resistant Aspergillus species and offers fungicidal activity against emerging fungal pathogens such as Scedosporium or Fusarium. All three azoles exhibit wide inter-individual variability in bioavailability, so monitoring of drug levels is required.
Product advantages
- 3PLUS1® Multilevel Calibrator Set available
- Tri-level controls available
- Tailor-made internal standard
This Chromsystems assay allows for reliable chromatographic determination of itraconazole, its active metabolite hydroxy-itraconazole, and posaconazole and voriconazole in only one isocratic HPLC run with fluorescence detection. Two very simple and efficient protein precipitation steps are used to separate interfering components and to obtain stable eluates. A tailor-made internal standard ensures reliable and precise quantification of the analytes.
These analytes - together with 9 further antimycotics - can also be determined using LC-MS/MS with the TDM Parameter Set Antimycotic Drugs from the MassTox® TDM Series A.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | Itraconazole/OH-Itraconazole: 0.03 mg/l |
| Upper Limit of Quantification | Itraconazole/OH-Itraconazole: up to 10 mg/l |
| Intraassay | CV = 1.1– 4.4 % |
| Interassay | CV = 2.4– 5.2 % |
| Recovery | 101–105 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | Itraconazole/OH-Itraconazole: 13 min; Posaconazole/Voriconazole: 8 min |
| Injection Volume | 20 µl (10 to 30 µl) |
| Flow Rate | 1.2 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | EX 261 nm EM 366 nm |
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. |
| Parameters | Hydroxyitraconazole, Itraconazole, Posaconazole, Voriconazole |
-
 Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Internal StandardOrder no.: 27044Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Internal StandardOrder no.: 27044Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precipitation Reagent 1Order no.: 27055Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precipitation Reagent 1Order no.: 27055Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precipitation Reagent 2Order no.: 27066Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precipitation Reagent 2Order no.: 27066Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precolumn Cartridge 4/10Order no.: 18027Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precolumn Cartridge 4/10Order no.: 18027Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC


Itraconazole
Hydroxyitraconazole
Posaconazole
Voriconazole
Clinical relevance
In recent years there has been an increase in invasive fungal infections due to the growing number of people with immunosuppression. Candida and Aspergillus are the most common types of infection, but previously rare fungal infections such as fusariosis or zygomycosis are also on the rise. Options for oral antimycotic therapy have improved considerably with the advent of new drugs. These include second-generation triazoles such as posaconazole and voriconazole.
Posaconazole is used primarily in case of therapy-resistant mycoses or drug intolerances. Voriconazole is also effective against some itraconazole-resistant Aspergillus species and offers fungicidal activity against emerging fungal pathogens such as Scedosporium or Fusarium. All three azoles exhibit wide inter-individual variability in bioavailability, so monitoring of drug levels is required.
Product advantages
- 3PLUS1® Multilevel Calibrator Set available
- Tri-level controls available
- Tailor-made internal standard
This Chromsystems assay allows for reliable chromatographic determination of itraconazole, its active metabolite hydroxy-itraconazole, and posaconazole and voriconazole in only one isocratic HPLC run with fluorescence detection. Two very simple and efficient protein precipitation steps are used to separate interfering components and to obtain stable eluates. A tailor-made internal standard ensures reliable and precise quantification of the analytes.
These analytes - together with 9 further antimycotics - can also be determined using LC-MS/MS with the TDM Parameter Set Antimycotic Drugs from the MassTox® TDM Series A.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | Itraconazole/OH-Itraconazole: 0.03 mg/l |
| Upper Limit of Quantification | Itraconazole/OH-Itraconazole: up to 10 mg/l |
| Intraassay | CV = 1.1– 4.4 % |
| Interassay | CV = 2.4– 5.2 % |
| Recovery | 101–105 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | Itraconazole/OH-Itraconazole: 13 min; Posaconazole/Voriconazole: 8 min |
| Injection Volume | 20 µl (10 to 30 µl) |
| Flow Rate | 1.2 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | EX 261 nm EM 366 nm |
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. |
| Parameters | Hydroxyitraconazole, Itraconazole, Posaconazole, Voriconazole |
-
 Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Internal StandardOrder no.: 27044Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Internal StandardOrder no.: 27044Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precipitation Reagent 1Order no.: 27055Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precipitation Reagent 1Order no.: 27055Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precipitation Reagent 2Order no.: 27066Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precipitation Reagent 2Order no.: 27066Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC -
 Precolumn Cartridge 4/10Order no.: 18027Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Precolumn Cartridge 4/10Order no.: 18027Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
Itraconazole/Posaconazole/Voriconazole Plasma Calibration StandardOrder no.: 27034Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
-
 HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC
HPLC Column Itraconazole, Posaconazole and Voriconazole in Serum/PlasmaOrder no.: 27110Itraconazole, Posaconazole and Voriconazole in Serum/Plasma - HPLC




